Broad-Spectrum Virucidal Activity of Nitric Oxide Nasal Spray (NONS) Against SARS-CoV-2 Variants and Major Respiratory Viruses (published January 9, 2026, https://www.mdpi.com/)
-
Broad-Spectrum Efficacy: Demonstrated potent virucidal activity against a wide range of respiratory pathogens, including SARS-CoV-2 (and its variants), Influenza A (H1N1, H3N2, H5N1), Influenza B, RSV, and Human Rhinovirus.
-
Rapid Inactivation: The study confirmed that Nitric Oxide Nasal Spray can neutralize 99.9% of viral loads within 15 seconds to 2 minutes of contact.
-
Effective Against Resistant Viruses: Successfully inactivated non-enveloped viruses like Rhinovirus (common cold), which are typically resistant to many conventional disinfectants and treatments.
-
Pandemic Preparedness: Highlights the role of NONS as a critical frontline intervention against emerging zoonotic threats, such as highly pathogenic avian influenza (H5N1).
Clinical Study - Effectiveness at Preventing COVID-19 After Exposure (published May 11, 2022, businesswire.com)
-
Clinical Study Suggests SaNOtize Nitric Oxide Nasal Spray Is Effective at Preventing COVID-19 after High-Risk Exposure
-
The new study, conducted at Srinakharinwirot University in Bangkok, Thailand (February 2022) found participants who took NONS after COVID-19 exposure were 75% less likely to become infected when compared to the control group, yielding a statistically significant reduction in infection rate.
-
After confirmed exposure to COVID-19, infection rate in participants (n=625) who took NONS™ was 6.4% versus 25.6% in the control group (P<0.0001).
-
The new data build upon previously reported Phase III trial results demonstrating that a rapid reduction of viral load prevents progression to infection after COVID-19 exposure.
Phase 3 Clinical Trial - 99% effectiveness against COVID -19 (published Feb 9, 2022)
- Phase 3 trial reached primary endpoint and demonstrated a reduction in SARS-CoV-2 log viral load in COVID-19 patients by more than 94% within 24 hours of treatment, and by more than 99% in 48 hours
- Patients in the Glenmark Phase 3 clinical trial in India self-administered a dose of 2 sprays per nostril, six times a day for a seven-day treatment period, along with standard supportive care.
- Treatment also demonstrated, in the high-risk group (n=218), a greater proportion of patients who achieved a combination of clinical and virological cure, based on the WHO Progression Scale
- Median time to virological cure was 4 days in the treatment group, 8 days in the placebo group (p < 0.05).
-
The study confirmed that SaNOtize’s Nitric Oxide Nasal Spray (NONS) represents a safe and effective antiviral treatment that shortens the course of COVID-19, and could prevent the transmission of COVID-19.
-
The reduction in log viral load corroborates the reduction of viral load in the UK Phase 2 trials (a reduction of 95% in 24 hours and 99% in 72 hours), conducted in March 2021 by Ashford and St Peter’s Hospitals NHS Foundation Trust, and Berkshire and Surrey Pathology Services, and published in the Journal of Infection in August 2021.
-
About the study: randomized, double-blind, placebo-controlled, parallel-arm study at 20 clinical sites across India that evaluated 306 patients.
Phase 2b early treatment trial was done in the UK, and NONS primary efficacy endpoint was achieved
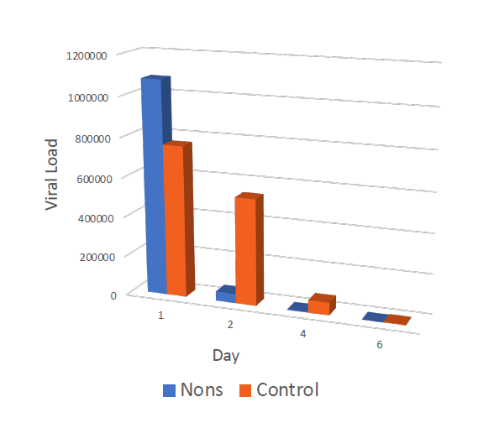
Double-blinded randomized early treatment for people tested Covid positive: 79 subjects (39 treatment, 40 placebo)
Regimen: self-administration of NONS 5-6x/day
The treatment group showed 16x higher viral reduction than the saline placebo arm on days 2 and 4, and 11x on day 6
47% of the treatment group felt better after four days of treatment vs. only 8% of the placebo group
Phase 2a prevention trial was done in Canada and showed high safety of NONS
Zero infections in the prevention arm of the trial
Insufficient number of infections in placebo arm to show statistically significant effectiveness
No significant adverse events even though the dose administrated using nasal lavage and gargle was much higher than nasal spray alone
In Vitro study
The company showed that NONS is highly efficient in vitro in immediately dropping the viral load of all tested Covid variants, including Delta, and other common viruses like influenza (H1N1).
Based on these results, SaNOtize says that this “invisible mask” is an excellent adjunct to vaccines, masks, hand sanitizer, and social distancing. The company believes that if Covid patients use NONS when the infection starts, the viral load will be much lower and, thus, symptoms will be lower.
The company reports it received approvals from Health Canada - no objection letter (NOL) - for two Phase 3 trials (prevention and early treatment), and it has started two Phase 3 treatment trials with their partners in India and Bahrain that will end by the end of 2021. The Canadian prevention trial has started. These trials will support full Health Canada Rx approval, followed by an Rx-to-OTC switch.
SaNOtize claims that its broad antiviral NONS can be a pivotal game-changer by providing an effective and convenient prophylactic solution that can be rapidly deployed with new viral strains and future pandemics. In addition to Covid, NONS can potentially prevent and treat other upper respiratory infectious diseases like influenza and the common cold. In countries which approved NONS as a medical device, the allowed claim is “to protect from viruses”.














